Part 3. Where does an Enzyme obtain energy? The ATP cycle
Welcome to BFSU: › Forums › Volume Three › Learning Progression B. Life Sciences › Lesson B-25. Anatomy and Physiology in Relation to Cells II: Principles of Metabolism › Part 3. Where does an Enzyme obtain energy? The ATP cycle
- This topic is empty.
-
AuthorPosts
-
-
October 24, 2022 at 4:02 pm #9096
Bernard Nebel
KeymasterReview and stress a cell’s continuous, huge need for energy.
- Synthesizing (making) materials requires energy. To grow, divide, and maintain itself, a cell must continually synthesize more proteins for enzymes, lipids for membranes, nucleic acids for DNA, etc. Things come apart on their own; to put them together, i.e. synthesize products, requires energy. (Note B from the preceding section: linking one amino acid to the next requires “a kick of energy” in addition to the enzyme.)
- The cell requires additional energy for whatever specialized task it is performing in the body, e.g. contraction of muscle fibers, conducting nerve impulses, making and secreting a digestive enzyme, etc.
From where does a cell get this energy?
Kids should already know, but review and stress as necessary. It comes form cell respiration: the oxidation of glucose.
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
But how is the energy given off from from the oxidation of glucose transferred to and actually drive the chemical reactions of synthesis, muscle contraction, conduction of nerve impulses, etc. (It is analogous to asking: How does the energy of burning gasoline get to turning the wheels of a car?)
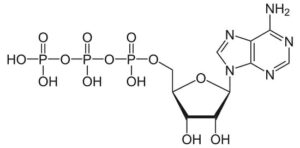
Studying energy-requiring chemical reactions occurring in cells, it was discovered that, in addition to the proper enzyme, a compound known by its initials as ATP (adenosine triphosphate) was also required. The molecular structure of ATP was worked out to be … (shown below, kids can identify the three phosphate groups of the tri-phospate.
The significant thing to know is that this molecule is like a “cocked spring”. The bond between phosphates the middle and end phosphates has very high potential energy. It is prone to break releasing a jolt of energy as the third phosphate group pops off. With the release of energy one is left the adenosine diphosphate (ADP) and a free phosphate group. In short: ATP. —-> ADP + P + Energy
In an actual reaction, such as linking two amino acids, an ATP attaches to the enzyme along with the reactants. The breakage of that P—O–P bond jolts the enzyme in such a manner that the desired bond forms the between the reactants. (The exact way this occurs is still not clear.)
This is known as a coupled reaction. The bonding of reactants, or other energy-requiring process, is coupled with the breakdown of ATP, i.e., ATP —> ADP. + P, which provides the required energy. The same is true for nearly all the energy requiring processes occurring in the cell. They are all coupled to and hence derive energy form the release of the “cocked spring” of ATP. That is ATP. —-> ADP + P
It should be self-evident that a cell goes through a lot of ATP in the course staying alive and performing its functions. Where does it get its ATP? Kids should be able to guess. It is from cell respiration.
The oxidation of glucose to carbon dioxide and water does not occur in a single “big bang”. There are many steps, each step catalyzed by an enzyme. In a number of these steps the reaction of breakdown is coupled with the regeneration of ATP. That is ADP + P —-> ATP. Yes, the C—C and C—H bonds of glucose being broken and combining with oxygen release more than enough to reform the P—O–P bond of ATP. (The many steps, the complete oxidation of one molecule of glucose releases sufficient energy to regenerate 36 molecules of ATP. There are no exceptions to the laws of thermodynamics.)
A major portion of glucose breakdown and ATP regeneration occurs within the tiny organelles called mitochondria within cells. (Find them in the cell diagram.) This is also where carbon atoms from glucose finally combine with oxygen to form carbon dioxide. Hence mitochondria and known as the “energy furnace” of cells.
The following video elaborates upon this ATP cycle. The video’s use of the term, “fermentation” may be confusing. Fermentation is a partial breakdown of glucose that occurs in the absence of oxygen. The end product is alcohol.
-
-
AuthorPosts
- You must be logged in to reply to this topic.